Why ESSEN-C™ Is Not Comparable to Over-the-Counter Vitamin C
At first glance, consumers and even some clinicians may wonder why ESSEN-C™ is priced higher than common sodium ascorbate powders found online or in health food stores. The answer is both straightforward and deeply scientific: ESSEN-C™ is not formulated to meet nutritional minimums — it is engineered to meet clinical expectations.
Purity and Potency: Built from the Start
What sets ESSEN-C™ apart is not just the final product, but the entire infrastructure behind it — a tightly controlled process that begins with narrowly defined raw material specifications and ends with a formulation trusted by leading integrative and regenerative medicine providers across the country.
Raw Material Integrity
ESSEN-C™ starts with non-GMO sodium ascorbate, manufactured in pharmaceutical-grade environments to proprietary internal specifications. These standards exceed those typically used in mass-market supplement production and ensure molecular identity, purity, and bioavailability.
Precision-Guided Formulation
ESSEN-C™ is processed through a proprietary, multi-phase manufacturing protocol that maintains molecular stability, ensures consistent pH (7.4–7.8), and delivers the fully ionized form of ascorbate required for effective physiological function.
Advanced Quality Control
Every batch undergoes multi-level third-party testing, including analysis by ISO 17025–certified laboratories operating under FDA-registered GMP, GLP, and GTP standards. Testing includes screening for:
- Microbial contaminants
- Heavy metals
- Mycotoxins
- Solvents and sulfur-based compounds
- Allergens and residual toxins
Post-Manufacturing Quarantine
No batch is released until it has passed comprehensive analytical validation confirming that it meets or exceeds criteria for purity, potency, and solubility.

Why This Matters in Clinical Settings
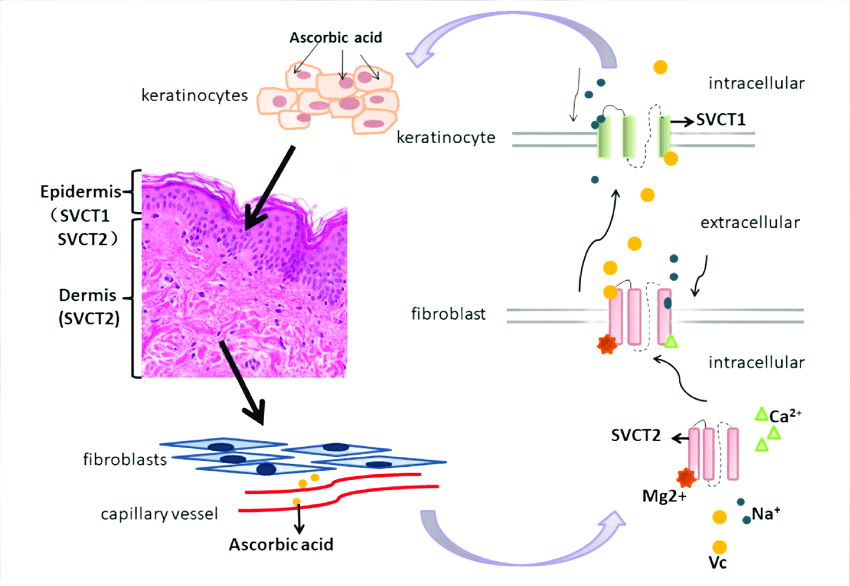
Sodium ascorbate is the active, ionized form of Vitamin C required by the body's SVCT1 and SVCT2 transporters for intracellular delivery — making bioavailability, molecular stability, and pH neutrality essential for clinical use.
Most retail products — even those labeled as sodium ascorbate — are:
- Produced under general cGMP (dietary supplement) standards
- Not held to pharmaceutical-adjacent manufacturing and testing protocols
- Lacking sourcing transparency and validation
- Formulated with hidden excipients, unstable pH, or poor solubility
- Inadequate for high-dose or terrain-sensitive protocols
ESSEN-C™ is designed for healthcare providers who require clinical-grade ascorbate that meets the needs of patients with oxidative burden, redox imbalance, mitochondrial dysfunction, or inflammatory conditions.
Because patients in clinical settings often present with complex metabolic stress, ESSEN-C™ supports protocols that prioritize:
- Redox homeostasis and glutathione cycling
- Mitochondrial and metabolic resilience
- Normal collagen formation and connective tissue repair
- Immune defense and antioxidant replenishment
- Compatibility with integrative interventions (e.g., peptides, ozone, biologics, and intravenous C protocols)

The Role of the SIP727® Protocol
ESSEN-C™ is the core formulation used in the SIP727® Protocol — a structured clinical approach for maintaining and sustaining physiological ascorbate levels between in-office therapies. This protocol helps optimize tissue saturation, support redox continuity, and extend the therapeutic impact of IV or adjunct therapies. Designed specifically for use under provider supervision, the SIP727® Protocol represents a growing clinical standard in terrain preparation and long-term patient care.